The Evolution of Metabolism
Metabolism refers to the chemical processes that occur within an organism to maintain life. These processes include the synthesis of molecules, the breakdown of molecules, and the exchange of matter and energy with the environment. The evolution of metabolism has played a crucial role in the development of life on Earth and the diversity of organisms that exist today.
The earliest forms of life on Earth are thought to have had simple metabolic processes, such as the synthesis of organic molecules from inorganic precursors. Over time, these processes became more complex and efficient, leading to the development of more advanced forms of metabolism.
One key step in the evolution of metabolism was the development of photosynthesis, which allowed some organisms to harness energy from sunlight to convert carbon dioxide and water into organic matter. This process, which is carried out by certain types of bacteria and algae, enabled these organisms to produce their own food and eventually gave rise to the oxygen-rich atmosphere we have today.
Other important developments in the evolution of metabolism include the emergence of cellular respiration, which allows organisms to extract energy from organic matter, and the development of the citric acid cycle, which is a key metabolic pathway that is involved in the breakdown of carbohydrates and fatty acids.
Overall, the evolution of metabolism has been a key factor in the diversification and success of life on Earth. By enabling organisms to harness and utilize energy from their environment, metabolism has allowed for the development of a wide range of life forms, each with its own unique adaptations and abilities.
Cells harvest the energy in chemical bonds
cells are able to harvest the energy stored in chemical bonds by breaking down molecules through a process called metabolism. This energy is used to power various cellular processes, such as synthesizing new molecules, carrying out mechanical work, and transporting substances across the cell membrane.
One way that cells harvest energy from chemical bonds is through the process of cellular respiration. This process involves the breakdown of glucose (a simple sugar) to produce energy in the form of ATP (adenosine triphosphate). During cellular respiration, the energy stored in the chemical bonds of glucose is released through a series of chemical reactions that occur in the cell's mitochondria.
Another way that cells harvest energy from chemical bonds is through the process of photosynthesis, which is carried out by certain types of bacteria and algae. During photosynthesis, light energy is used to convert carbon dioxide and water into glucose, releasing oxygen as a byproduct. This process allows these organisms to produce their own food and is a key source of energy for many other organisms as well.
Overall, the ability to harvest energy from chemical bonds is a key characteristic of life and is essential for the survival and growth of cells and organisms.
Using Chemical Energy to Drive Metabolism
Chemical energy is a type of energy that is stored in the bonds between atoms and molecules. It is the energy that is released or absorbed when chemical reactions occur. In living organisms, chemical reactions are used to drive metabolism, which is the process of converting energy from food into usable forms of energy for the body's cells.
For example, the process of cellular respiration is a chemical reaction that occurs in cells to convert glucose (a sugar) into ATP (adenosine triphosphate), which is the primary source of energy for cells. ATP is produced through a series of chemical reactions that involve the breakdown of glucose and the release of energy. This energy is then used by cells to perform various functions, such as muscle contraction, transport of molecules, and synthesis of biomolecules.
Other chemical reactions in the body, such as those involved in the synthesis of proteins, also require energy. These reactions are driven by the energy that is released when chemical bonds are broken or formed during the reaction.
Overall, chemical energy plays a central role in the metabolism of living organisms, as it is used to power the many chemical reactions that are necessary for the proper functioning of the body.
Cellular respiration oxidizes food molecules
that's correct! Cellular respiration is a process that occurs in cells to convert the energy from food molecules into a form that cells can use. This process involves the oxidation of food molecules, such as glucose, to release energy.
During cellular respiration, glucose is broken down in a series of chemical reactions that take place in the mitochondria, the energy-producing factories of the cell. These reactions involve the transfer of electrons from glucose to oxygen, resulting in the production of ATP (adenosine triphosphate), which is the primary source of energy for cells.
The process of cellular respiration can be broken down into three main stages: glycolysis, the citric acid cycle (also known as the Krebs cycle), and the electron transport chain. Each of these stages involves a series of chemical reactions that are powered by the energy released from the breakdown of glucose.
Overall, cellular respiration is an important process that allows cells to convert the energy from food molecules into a usable form, enabling the body to perform various functions such as muscle contraction and the synthesis of biomolecules.
Glucose Catabolism
Glucose catabolism is the process by which cells break down glucose, a simple sugar, to produce energy. This process occurs in all cells, but it is especially important in cells that have high energy demands, such as nerve cells and muscle cells.
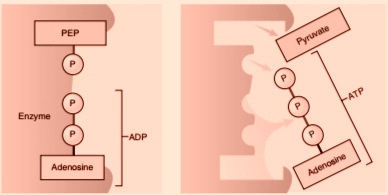
The breakdown of glucose is a complex process that involves several steps. The first step is glycolysis, which occurs in the cytoplasm of the cell. During glycolysis, glucose is broken down into two molecules of pyruvate, a process that releases energy in the form of ATP (adenosine triphosphate).
If oxygen is present, the pyruvate produced during glycolysis is further broken down through a process called aerobic respiration. During aerobic respiration, the pyruvate is converted into acetyl-CoA, which enters the mitochondria and is used in the citric acid cycle (also known as the Krebs cycle). The citric acid cycle produces ATP, NADH, and FADH2, which are used to produce energy through the electron transport chain.
If oxygen is not present, the pyruvate produced during glycolysis is converted into lactic acid through a process called anaerobic respiration. This process also produces ATP, but at a lower efficiency compared to aerobic respiration.
Overall, glucose catabolism is an important process that allows cells to produce energy to carry out their various functions. Dysregulation of glucose catabolism can lead to various health problems, such as diabetes and obesity.
Stage One: Glycolysis
Glycolysis is the first stage of glucose catabolism and occurs in the cytoplasm of the cell. It involves the breakdown of glucose into two molecules of pyruvate and the production of ATP and NADH.
Glycolysis can be divided into two stages: the energy-investment phase and the energy-payoff phase.
During the energy-investment phase, two ATP molecules are used to convert glucose into a six-carbon molecule called fructose 1,6-bisphosphate. This molecule is then split into two three-carbon molecules called glyceraldehyde 3-phosphate.
During the energy-payoff phase, each glyceraldehyde 3-phosphate molecule is further broken down into two molecules of pyruvate, producing ATP and NADH in the process. In total, glycolysis produces a net gain of two ATP and two NADH molecules per glucose molecule.
Glycolysis is an important process that occurs in all cells, but it is especially important in cells that have high energy demands, such as nerve cells and muscle cells. It is also an important process for the production of energy in the absence of oxygen, such as during exercise. Dysregulation of glycolysis can lead to various health problems, such as diabetes and obesity.
Stage Two: The Oxidation of Pyruvate
The oxidation of pyruvate is the process by which pyruvate, a three-carbon molecule produced during glycolysis, is further broken down to produce energy. This process occurs in the mitochondria of the cell and is divided into two stages: the conversion of pyruvate into acetyl-CoA and the citric acid cycle (also known as the Krebs cycle).
During the conversion of pyruvate into acetyl-CoA, pyruvate is converted into acetyl-CoA through a process called pyruvate decarboxylation. This process involves the removal of a carbon dioxide molecule from pyruvate, producing acetyl-CoA in the process. The conversion of pyruvate into acetyl-CoA also requires the presence of oxygen and the enzyme pyruvate dehydrogenase.
The citric acid cycle (Krebs cycle) is a series of chemical reactions that occurs in the mitochondria of the cell and involves the breakdown of acetyl-CoA to produce ATP, NADH, and FADH2. The citric acid cycle begins with the conversion of acetyl-CoA into a six-carbon molecule called citrate. Citrate is then broken down into a series of smaller molecules, producing ATP, NADH, and FADH2 in the process.
The oxidation of pyruvate is an important process that occurs in all cells, but it is especially important in cells that have high energy demands, such as nerve cells and muscle cells. It is also an important process for the production of energy in the presence of oxygen. Dysregulation of the oxidation of pyruvate can lead to various health problems, such as diabetes and obesity.
Stage Three: The Krebs Cycle
The citric acid cycle, also known as the Krebs cycle, is a series of chemical reactions that occurs in the mitochondria of the cell and involves the breakdown of acetyl-CoA to produce ATP, NADH, and FADH2. The citric acid cycle is the second stage of glucose catabolism, following the conversion of pyruvate into acetyl-CoA.
The citric acid cycle begins with the conversion of acetyl-CoA into a six-carbon molecule called citrate. Citrate is then broken down into a series of smaller molecules, producing ATP, NADH, and FADH2 in the process. The citric acid cycle involves a series of enzyme-catalyzed reactions, including decarboxylation, dehydration, and hydration reactions.
During the citric acid cycle, two ATP molecules are produced per acetyl-CoA molecule, as well as three NADH and one FADH2 molecule. The NADH and FADH2 molecules produced during the citric acid cycle are used to produce energy through the electron transport chain.
The citric acid cycle is an important process that occurs in all cells, but it is especially important in cells that have high energy demands, such as nerve cells and muscle cells. It is also an important process for the production of energy in the presence of oxygen. Dysregulation of the citric acid cycle can lead to various health problems, such as diabetes and obesity.
Harvesting Energy by Extracting
Electrons
There are several ways to harvest energy by extracting electrons from a material or system. Here are a few examples:
Photovoltaics: Photovoltaics is the process of converting sunlight into electricity using solar cells. Solar cells are made of semiconductor materials, such as silicon, that absorb photons of light and release electrons, generating an electric current.
Electrochemical reactions: Electrochemical reactions involve the transfer of electrons between a cathode and an anode in an electrochemical cell. For example, a battery converts chemical energy into electrical energy through an electrochemical reaction between the cathode and the anode.
Thermoelectric generation: Thermoelectric generation involves the conversion of heat into electricity using thermocouples. Thermocouples are made of two different conductive materials that produce a voltage when a temperature gradient is applied across them.
Electromagnetic induction: Electromagnetic induction is the process of generating an electric current by moving a conductor through a magnetic field. This is the principle behind generators, which convert mechanical energy into electrical energy.
Piezoelectricity: Piezoelectricity is the electric charge that accumulates in certain solid materials, such as quartz, when subjected to mechanical stress. Piezoelectric materials can be used to generate electricity from motion, such as footsteps or vibrations.
Stage Four: The Electron Transport Chain
The electron transport chain is a series of biochemical reactions that occur in the mitochondria of cells. It is the final stage of aerobic cellular respiration, in which the energy stored in glucose and other organic molecules is converted into ATP, the energy currency of the cell.
In the electron transport chain, electrons are transferred from organic molecules to oxygen through a series of protein complexes and electron carriers, such as NADH and FADH2. As the electrons are transferred, energy is released and used to pump protons across the inner mitochondrial membrane, generating a proton gradient. This proton gradient is used to power the synthesis of ATP through the process of chemiosmosis.
The electron transport chain is also known as the respiratory chain or oxidative phosphorylation. It is a vital process for the production of energy in cells, and it is necessary for the survival and function of most living organisms.
Summarizing Aerobicn Respiration
Aerobic respiration is a metabolic process that occurs in the mitochondria of cells and involves the breakdown of glucose and other organic molecules to produce energy in the form of ATP. It occurs in four stages:
Glycolysis: This occurs in the cytoplasm of cells and involves the breakdown of glucose into two molecules of pyruvate. Some ATP is produced, and the electron-carrying molecule NADH is produced.
The pyruvate dehydrogenase complex: Pyruvate is converted into acetyl CoA, and NADH is produced.
The citric acid cycle (also known as the Krebs cycle): Acetyl CoA is broken down further, producing more NADH and FADH2.
The electron transport chain: Electrons from NADH and FADH2 are transferred to oxygen, generating a proton gradient across the inner mitochondrial membrane. This gradient is used to produce ATP through chemiosmosis.
Overall, aerobic respiration is a highly efficient process for producing energy, as it can produce up to 36 ATP molecules from one molecule of glucose. It requires oxygen to occur and generates carbon dioxide as a byproduct.
Regulating Aerobic Respiration
There are several mechanisms that regulate aerobic respiration in cells:
Enzyme activity: Many enzymes involved in aerobic respiration are regulated by allosteric modulation, in which the activity of the enzyme is increased or decreased by a molecule binding to a specific site on the enzyme. For example, the enzyme pyruvate dehydrogenase is inhibited by high levels of ATP and activated by low levels of ATP.
Hormonal regulation: Hormones, such as insulin and glucagon, can regulate aerobic respiration by altering the rate at which glucose is taken up and used by cells.
Gene expression: The expression of genes involved in aerobic respiration can be regulated by transcription factors, which bind to specific DNA sequences and control the transcription of a gene into RNA.
Mitochondrial biogenesis: The number and size of mitochondria in a cell can be regulated in response to changes in energy demand.
Caloric restriction: Caloric restriction, or reducing caloric intake, can increase the efficiency of aerobic respiration and extend lifespan by reducing oxidative stress and increasing the production of ATP.
Overall, the regulation of aerobic respiration is complex and involves the coordinated actions of various enzymes, hormones, transcription factors, and other cellular mechanisms.